| Name | HCV Ab ELISA Test |
| Full name | Human Hepatitis C Virus ELISA Test Kit, Export Use Only |
| Category Name | Hepatitis ELISA kits |
| Test | 96, |
| Method | ELISA method: Enzyme Linked Immunosorbent Assay |
| Principle | Double Antigen Sandwich ELISA |
| Detection Range | Qualitative: Positive & Negative Control |
| Sample | 50 µl |
| Sensitivity | 99.8% |
| Total Time | ~ 120 min |
| Shelf Life | 12 Months from the manufacturing date |
Anti-HCV ELISA Kit description
The HCV Ab ELISA Test is for clinical lab diagnosis of patients who are suspected of having a hepatitis C virus infection, and for blood donor screening. This HCV Ab ELISA test is an enzyme-linked immunosorbent assay for in vitro qualitative identification of IgG antibodies to hepatitis C virus in human serum/plasma.
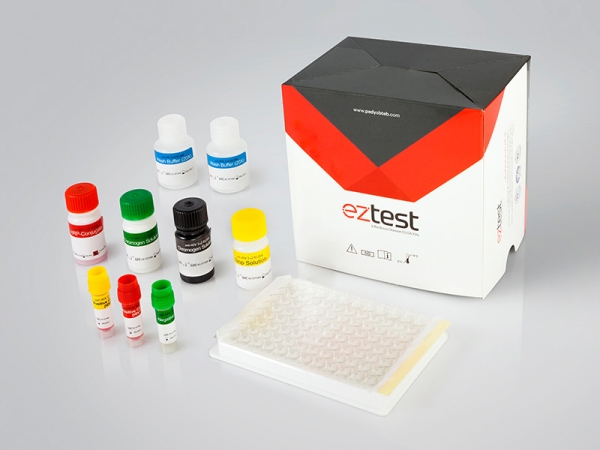
Materials Provided with HCV Ab ELISA test kit:
1. Microwell Plate: each well contains recombinant HCV antigens
2. Negative Control: Protein-stabilized buffer tested non-reactive for HCV antibodies
3. Positive Control: Anti-HCV antibodies diluted in protein-stabilized buffer.
4. HRP-Conjugated Reagent: HRP-conjugated rabbit anti-human IgG antibodies
5.BIOTIN-Conjugate
6. Wash Buffer: PH 7.4, 20X PBS
7. Chromogen Solution A: Urea peroxide solution.
8. Chromogen Solution B: TMB solution
9. Stop Solution: Diluted sulfuric acid solution (0.5M H2SO4)
Materials Required, not Provided:
1. Precision pipettes
2. Distilled or deionized water
3. EIA kit Microplate Washer
4. EIA kit Microplate Reader with a 450 nm